Introduction
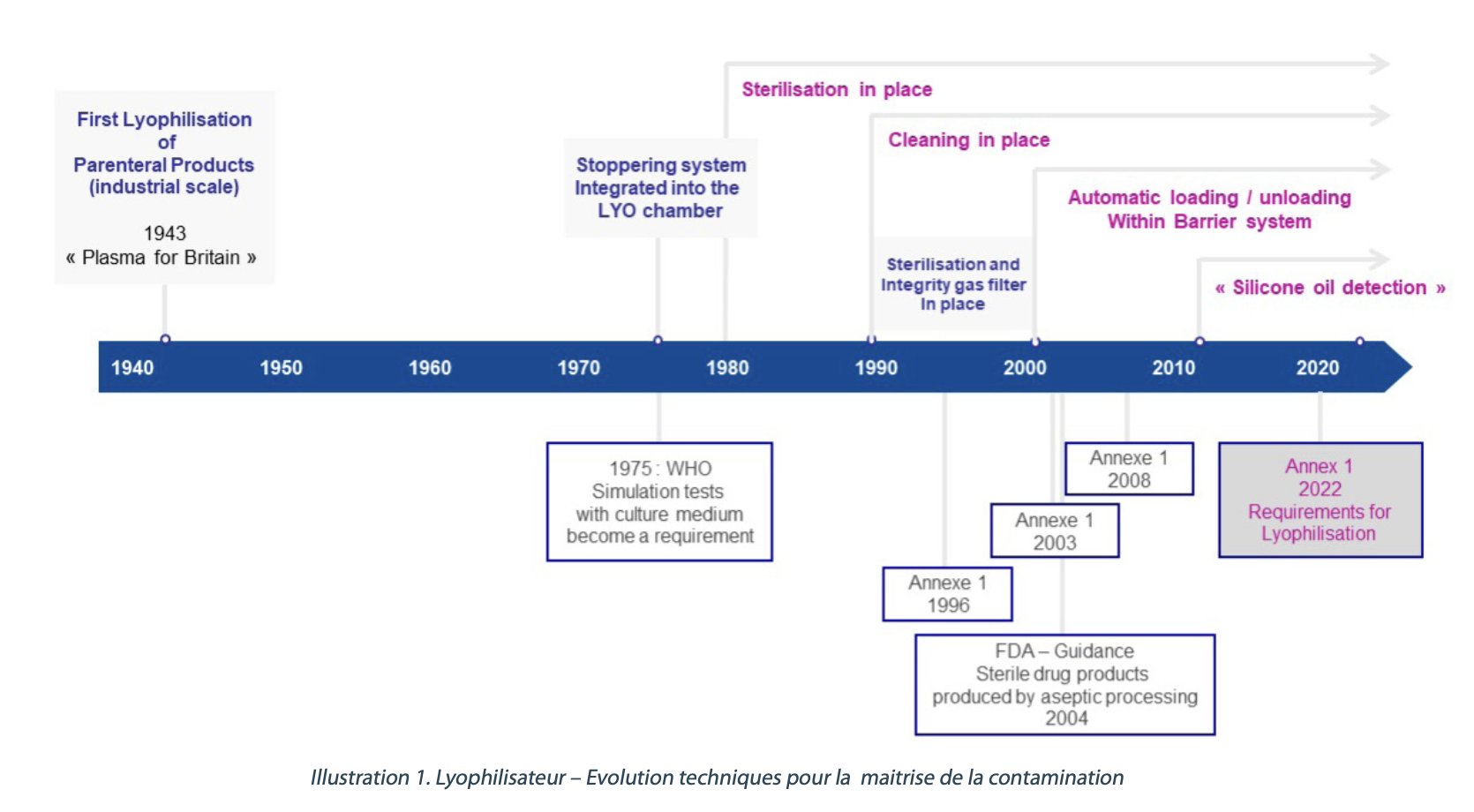
Pharmaceutical regulatory standards and industry best practices are constantly evolving as demand for sterile drug production capacity grows. Annex 1 is the international reference text for the latest standards in sterile drug manufacturing. It encourages the use of innovative technologies, including barrier technologies, which are becoming essential. Appendix 1 also details the requirements for freeze-drying/freeze dryers more precisely and opens the door to new strategies.
While Annex 1 makes multiple references to “barrier technologies” with the main objective of reducing direct interactions between sources of contamination and aseptic processes, the requirements for interfaces with freeze dryers remain somewhat vague, but represent a real challenge at the industrial level. This is why the “Barrier Technologies” and “Freeze-Drying” GICs have decided to join forces for this event.
During these A3P Technologies Barriers and Freeze Drying 2025 days, we will address the future challenges facing our industry through technical and regulatory topics and provide answers through practical applications:
- Regulatory update
- Managing weak points: ergonomics, transfers, integrity/glove management
- Challenges for isolator set-up of indirect product contact surfaces (parts in contact with caps)
- Specific features related to RABS
- Use of new technologies in the design phases (augmented/virtual reality)
- Campaign mode on a freeze dryer with automatic loading under a technological barrier
- Challenges at the isolator/freeze dryer interfaces
- Issues related to biological indicators and prospects with enzymatic indicators
And many other topics presented by two enthusiastic GICs!
Official conference language: French
Simultaneous translation: English